Hardware Solutions
Applications
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Immunology
Dr. Ken Coppieters and Dr. Mathias von Herrath, La Jolla Institute for Allergy & Immunology
Researchers at the La Jolla Institute for Allergy & Immunology recently used intravital two-photon imaging and Imaris to create and analyze real-time movies of the cellular process underlying type 1 diabetes in mouse models. This new view provides insights into the autoimmune process that leads to type 1 diabetes and could influence future therapies.
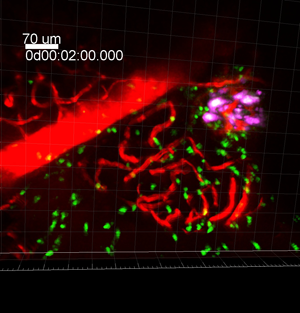
Figure 1: This two-photon microscopy image shows part of a time series in which diabetogenic CD8 T cells (green), beta cells (magenta), and local vasculature (red) are seen in the pancreas.
Type 1 diabetes occurs when the body’s immune system destroys insulin-producing beta cells in the pancreas, but it has been challenging to image this process in action. The researchers performed intravital two-photon microscopy using a new procedure developed by Dr. Matthias von Herrath to surgically expose the pancreases of living mice.
“We were advised that Imaris is the best available software solution for automatic tracking and 4D visualization of image sequences captured by two-photon microscopy,” says the paper’s first author Dr. Ken Coppieters, who is formerly of the La Jolla Institute and now at Ghent University in Belgium.
The researchers first used Imaris for 3-D isosurfacing analysis of vascular perfusion patterns and vascular leakage. They found that control animals exhibited vascular integrity while the prediabetic animals had leaky vessels that allow T cells to move from the blood stream to the pancreas where they kill the beta cells.
They then conducted longitudinal 3-D tracking of T cells using Imaris. Measurements of the T cell velocity, directionality and arrest parameters revealed how these cells can launch an attack and in particular elucidated the time sequence of events during the destruction of beta cells within the pancreas of mice. The researchers found that the T cells move randomly throughout the pancreas until they encounter beta cells. They then slow down and release toxic substances that kill beta cells in a process that takes several hours. Imaris, Imaris Measurement Pro and Imaris Track were used to conduct this work.
The analysis also showed that it took tens of millions of T cells to produce massive beta cell destruction, a finding that provides evidence that the autoimmune attack is occurring for years before the disease is diagnosed. In humans, usually 90 percent of beta cells are destroyed before the disease produces enough symptoms to be recognized. “From a therapeutic perspective, these studies suggest that we may need to find a way to prevent the T cells from accessing the pancreas in the first place, since once they do, they have the ability to destroy several beta cells at a time,” Dr. Coppieters says.
T cells (purple dots) attack the pancreatic islets (green images in center), which contain the insulin-producing beta cells. The squiggly "tail" on the T cells, which switches from blue to red to yellow, is a tracking mechanism inserted by the researchers to record the T cell's velocity and attack time. Credit: Video by La Jolla Institute for Allergy & Immunology. Published by the Journal of Clinical Investigation.
Research Paper: Coppieters K, Amirian N, von Herrath M. Intravital imaging of CTLs killing islet cells in diabetic mice J Clin Invest. 2012 Jan 3;122(1):119-31. doi: 10.1172/JCI59285.
