Hardware Solutions
Applications
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Cancer Case Study
Nicholas Kurniawan from the FOM Institute AMOLF in Amsterdam and researchers from the National University of Singapore are using Imaris to understand how the extracellular matrix in which cancer cells spread can affect pharmacological treatments. This research could lead to better therapies for preventing cancer metastasis, the leading cause of cancer mortality.
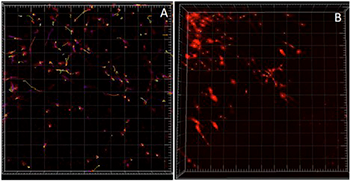
Movement of individual MDA-MB-231 malignant breast cancer cells in a collagen gel. Time-lapse, three-dimensional confocal fluorescence images of the cells (red) were analyzed using Imaris to detect the cells, quantitatively localize their positions in each frame, and construct the complete migration trajectories. This example shows the 3D cell tracks during 8 hours of migration in a 2.5 mg/ml collagen gel formed at pH 7.4, (A) before and (B) after treatment with cytochalasin D. (B) shows little or no movement of cells as compared to (A). Tracks are color-coded for time.
Various studies have shown that the physical and mechanical properties of the 3D extracellular matrix can affect a large variety of cell behaviors, including cancer cell invasion. Based on this knowledge, the researchers hypothesized that different pharmacological approaches may be more effective in certain matrices or tumor microenvironments.
To test their hypothesis, the investigators developed a collagen hydrogel model with tunable mechanical properties in which they could monitor the three-dimensional invasion of highly malignant breast cancer cells. The researchers turned to Imaris because of its powerful cell-tracking algorithm, which allowed them to accurately locate each cell and reliably reconstruct cell trajectories. “The visual display makes it very easy to see the tracked objects and the software also enables semi-automated tracking, which allows us to correct some misidentified objects,” said Kurniawan.
Watching cancer invade
The researchers acquired time-lapse confocal fluorescence Z-stacks (647 X 647 X 100 microns) of the collagen hydrogel after the cancer cells were given a chance to invade. They used the Imaris tracking algorithm to follow cells in the presence and absence of drugs that affect four typical mechanisms of cell migration in five different extracellular matrices with varying biophysical properties. They discarded cell tracks shorter than 3500 s to eliminate random and system noise and corrected tracks for overall drift when necessary.
For the extracellular conditions tested by the researchers, cancer cell motility showed a weak dependence on matrix mechanics without drug treatment. They also found that the effectiveness of anti-migratory drugs was dependent on the biophysical conditions of the three-dimensional matrix.
“Our study not only confirmed our hypothesis, but also provided a strong indication that cancer cells have a remarkable ability to actually adapt their migrational machineries depending on the physical characteristics of the environment,” said Kurniawan. “Our findings also offer concrete guidelines for selecting the more effective pharmacological approaches for different biophysical conditions of the tumor, and our experimental model provides a practical yet powerful way to test drug effectiveness in 3D matrices with different structural or mechanical properties.”
The researchers are expanding this study to examine not only cultured cancer cells, but also circulating tumor cells (CTCs), which can be found circulating in the blood stream, ready for the next step of metastasis. They have isolated these cells from patients using a newly developed microfluidics technique that preserves their viability and examined cell migration behavior in different matrices using Imaris. Since these cells are really the “metastatic seeds” in distant organs, they hope to better understand and eventually limit the migration of these cells in physiologically relevant matrices.
Browse our range of related assets below...
Author: Wei Sun and Chwee Teck Lim, National University of Singapore, Nicholas A. Kurniawan, FOM Institute A
Category: Case Study
