Hardware Solutions
Applications
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Neuroscience
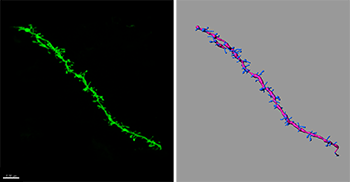
Using Imaris, the researchers reconstructed dendritic spines (right) from confocal images (left), which revealed that APC deletion lead to significant increases in spine density along apical dendrites of pyramidal neurons in both the cortex and hippocampus. Image and 3D reconstruction generated by Dr. Antonella Pirone.
Dr. Michele Jacob from Tufts University in Boston is leading a team of researchers using Imaris to better understand how a mutant gene affects a neurological pathway involved in intellectual disability and autism spectrum disorder. Treatment strategies for these conditions are lacking because the underlying molecular causes are poorly understood. This research could lead to the discovery of new molecular targets for therapeutic interventions.
Studies have suggested that the hundreds of risk genes involved in intellectual disability and autism converge on a few key biological processes in neurons. The investigators are focusing on one of these processes, known as the β-catenin/canonical Wnt signaling pathway. They are studying the adenomatous polyposis coli (APC) protein, which is the major negative regulator for this pathway. APC’s role in brain development, function, learning, and behavior is poorly defined.
“To gain insights into APC’s function, we have generated a new mouse model with targeted deletion of APC in neurons during synaptic differentiation,” says Dr. Jacob. “Our APC conditional knockout mice, compared with wild-type littermates, display delayed learning, poor memory formation, and autistic-like disabilities (reduced social interest, increased repetitive behaviors).”
The researchers used Imaris software to test whether loss of APC and the higher levels of β-catenin that come with this loss affect the density and structural maturation of dendritic spines. “Imaris software offered the best approach for 3D reconstruction of dendritic spines visualized by crossing our mutant mice with GFP transgenic mice,” says Dr. Antonella Pirone, a postdoctoral fellow in Jacob’s lab. “The software provides versatility and reliability; we could adapt the same algorithm to many images of neurons from test and control mice for accurate quantitative comparisons of spine density in 3D.”
To determine dendritic spine density, the researchers acquired confocal microscopy images of neurons from the mutant/GFP transgenic mice as well as control mice. They then used Imaris Filament Tracer to track, edit, draw, display, measure, and reconstruct dendritic filaments and spines. They used this information to calculate the density and shape of the spines.
The analysis revealed that APC deletion led to significant increases in spine density along apical dendrites of pyramidal neurons in both the cortex and hippocampus. These structural changes were consistent with functional, molecular, and behavioral alterations seen in the mutant mice but not in wild-type littermates.
The researchers are now extending their spine density analysis to other mutant mouse models of intellectual and autism-like disabilities. They are also evaluating the therapeutic effectiveness of pharmacological treatments at different developmental stages. “Accurate quantification of spine density changes in multiple mouse models, both in the presence versus absence of pharmacological treatments, provides an important approach for assessing structural features of neurons that likely impact function at the synaptic and circuit levels,” says Dr. Jacob.
