Hardware Solutions
Applications
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Neuroscience
The crustacean stomatogastric nervous system (STNS) controls the movement of the organism’s stomach muscles and is often used as a model to study a network of neurons that produces a motor pattern. Researchers led by Dr. Marie L. Goeritz of Brandeis University in Massachusetts used Imaris software to develop a new masking method that allowed them to gain a detailed understanding of neuron morphology in the stomatogastric nervous system.
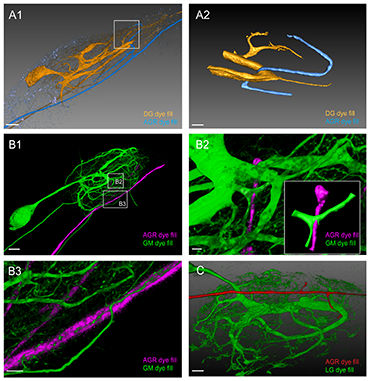
Double fills of the AGR and other STG neurons identify potential synaptic partners. A1. Double fill of the dorsal gastric (DG) neuron with tetramethylrhodamine-dextran (TMR, yellow) and the AGR neuron with Lucifer Yellow (LY, blue) shows candidate contact sites in the neuropil. Lateral blend mode projection of 18 merged confocal image stacks, each consisting of 133 optical slices (acquired at resolution of 0.177 x 0.177 x 1.007 micrometers). Scale bar is 40 micrometers. A2. Reconstructed surface visualization of the DG-AGR candidate contact site in the coarse neuropil, reconstructed from the same data set as A1. Scale bar is 10µm. B1. Double fill of a GM neuron with neurobiotin (green) and the AGR neuron with alexa Fluor 594-hydrazide (magenta). Blend mode projection of 18 merged confocal image stacks, each consisting of 185 optical slices (acquired at resolution of 0.088 x 0.088 x 0.504 micrometers), background-subtracted. Scale bar is 50µm. B2. Close-up of candidate contact site in the coarse neuropil. Scale bar is 5µm. A reconstructed surface visualization of the contact site (inset in B2) allows a clearer view. B3. Close-up of candidate contact site between fine neuropil processes of gastric mill neurons (GM) and the AGR axon. Scale bar is 10 micrometers. C. Double fill of the lateral gastric neuron (LG) neuron with LY (green) and the AGR neuron with alexa Fluor 594-hydrazide (red) shows apparent contact site between the LG primary neurite and an AGR process. Blend mode projection of 8 merged confocal image stacks, each consisting of 155 optical slices (acquired at resolution of 0.177 x 0.177 x 0.336 micrometers), background-subtracted. Scale bar is 30 micrometers. Reprinted from Goeritz ML, et al. PLoS One 8(12): e79306.
The crustacean STNS includes a muscle receptor neuron called the anterior gastric receptor (AGR), which provides feedback that influences the activity of motor circuits in the stomatogastric ganglion (STG) through polysynaptic pathways. Although the crustacean STNS has been well studied, the new image analysis approach revealed projections that had never before been observed in the stomatogastric ganglion.
“We used Imaris because it allowed us to perform segmentation and reconstruction of high-resolution stacks – and, therefore, very large files - on one platform,” said Dr. Goeritz. “Further, Imaris was useful in using the masking technique without having to write too much software on our own.”
The researchers studied the AGR within the STNS of a Jonah crab. They began by filling the somata or axons of neurons with fluorescent dye and then collected stacks of confocal images of the AGR. They used Imaris Slice and Surpass views to adjust contrast and brightness and to display stacks as maximum-intensity projections or as blend mode projections. Displaying subsets of the z-stacks as blend mode projections with reduced opacity improved visualization of cell fills and protein distribution. To determine branch lengths and branch volumes, the researchers used the Filament module to manually make volume reconstructions of cell fills.
Determining pre- or post-synaptic sites
The researchers developed a masking method using the Create mask functionality to establish whether patches of synapsin-like labeling were likely pre- or post-synaptic sites in the AGR neuron. Synapsin is a protein that regulates neurotransmission and is found at the presynaptic site.
“Usually confocal microscopy cannot clearly separate pre- and postsynaptic sites,” said Dr. Goeritz. “However, our method of using surface reconstructions as masks to filter the synapsin signal detected any voxel of synapsin-like signal that overlapped with filled cells. This let us separate the signal into putative pre- and post-synaptic sites.”
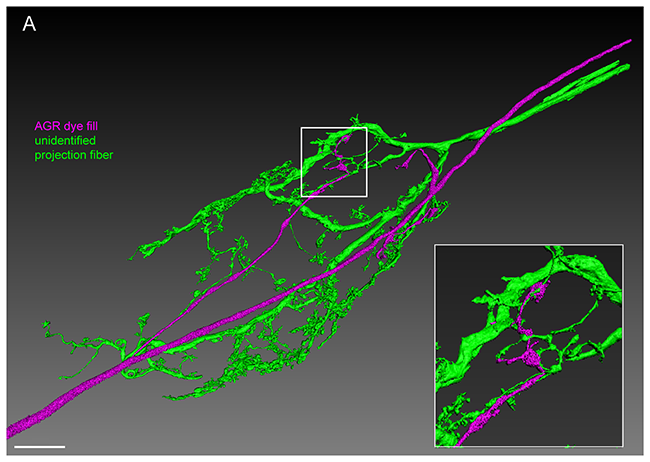
The AGR projections are in close apposition with projections from descending fibers in the STG neuropil. Double fill of an unidentified STN process with LY (green) and the AGR neuron with alexa Fluor 594-hydrazide (magenta) shows close apposition of processes over a large area in the STG neuropil. Reconstructed surface visualization of 9 merged confocal image stacks, each consisting of 229 optical slices (acquired at resolution of 0.168 x 0.168 x 0.252 micrometers). Scale bar is 50 micrometers. Insert shows close-up of boxed area, revealing apparent contact sites between the AGR projection terminals and fine processes of the unidentified projection neuron. Reprinted from Goeritz ML, et al. PLoS One 8(12): e79306.
For the masking method, the researchers first used surface reconstructions of the filled cell as a mask to isolate synapsin-like immunoreactive labeling within filled processes from the overall synapsin-like signal. They did this by using the Imaris Surface module to create surface reconstructions using a manually set intensity threshold to distinguish between background and cell fill. The overlapping area for the reconstructed cell surface mask and the synapsin-like immunoreactive signal was saved as a new signal channel that they called “synapsin-inside.”
The investigators then captured synapsin-like labeling adjacent to the cell by repeating the same process but this time they used a lower threshold of intensity. This captured the “glow” around the cell surface and created a mask that over-estimated the volume of the cell. They isolated synapsin-like labeling in the vicinity of the filled cell by using the Imaris Channel Arithmetics function to subtract the “synapsin-inside” signal from this new signal channel. The researchers classified synapsin-like immunoreactivity that was observed only within the AGR as pre-synaptic and patches of synapsin-like labeling seen in the wider volume reconstruction of AGR as postsynaptic.
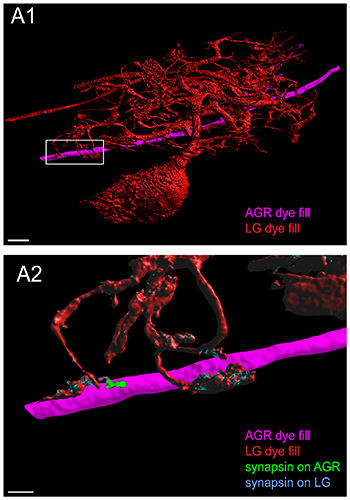
Putative chemical synapses at a contact site between LG and AGR. A1. Double fill of the LG neuron with LY (red) and the AGR neuron with alexa Fluor 594-hydrazide (magenta) shows apparent contact sites of LG processes wrapping around very short stubby projections on the AGR axon. A2. Higher magnification of the box in A1 shows adjacent patches of synapsin immunoreactivity in the LG and in the AGR neuron at their contact site. The image was processed to show synapsin labeling in the LG neuron in blue and synapsin labeling in the AGR neuron in green. A1 and A2 are reconstructed surface visualizations of the same data set of 12 merged confocal image stacks, each consisting of 182 optical slices (acquired at resolution of 0.158 x 0.158 x 0.504 micormeters). Reprinted from Goeritz ML, et al. PLoS One 8(12): e79306.</class="content-image" p>
The masking method revealed a non-uniform distribution of pre- and post-synaptic sites, with distinct clusters in the filled area of the AGR. Overall, the image analysis showed that AGR processes were diverse in terms of shape, number of branches and branching structure. The number of projections in the AGR ranged from one to three simple projections to multiple branched processes.
Dr. Goeritz says that the fact that the AGR not only has processes in the neuropil, but that these processes are likely sites of synaptic interaction, means that sensory feedback might play an important role in regulating the activity of this network and warrants further investigation. She notes that a previous, very thorough, electron microscopy study of the homolog and remarkably similar STG in the Spiny lobster Panulirus Interruptus (King et al. 1976a) did not show any processes of the AGR neuron within the STG, implying that they might be an interesting feature of taxa differentiation.
“From a technical point of few, electron microscopy study is still the gold standard and would be needed for ultimate proof that these putative synapses are real, but our method is great as a first pass screen and allows a relatively quick evaluation of putative synapse locations without the costs and expertise required to perform electron microscopy studies,” says Dr. Goeritz.
Research Paper: Goeritz ML, Bowers MR, Slepian B, Marder E (2013) Neuropilar Projections of the Anterior Gastric Receptor Neuron in the Stomatogastric Ganglion of the Jonah Crab, Cancer Borealis. PLoS ONE 8(12): e79306. doi:10.1371/journal.pone.0079306
Author: Dr. Marie L. Goeritz and colleagues, Brandeis University
Category: Case Study
