Hardware Solutions
Applications
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Cancer Research
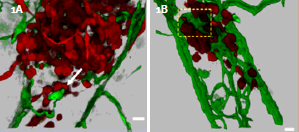
A)3D recontruction of MDA-435 cell microtumor, interacting with remodeling vessels. B)3D reconstruction of of MDA-435 tumor cells secreting VEGF in the zebrafish bodywall. See interactive movie 3 and 4of 3D reconstructions of digitally isolated tumor cells using ImarisContoursurfacefunction cells in contact with host vessels from B) and C). Fish bloodvessels=green, tumor cells=red; scale bars= 20μm.
Metastasis, the major cause of death in cancer patients, is a highly dynamic process that occurs in multiple steps, which include disruption of cell-cell adhesion, migration of cells away from the primary tumor and intravasation into the vasculature
Understanding this process has been limited by the inability of existing animal models (mouse, chicken) to visualize tumor cell behavior in real time. Recently, researches in the lab of Professor Klemkeat UCSD have developed a novel translucent zebrafish xenograft model of human cancer progression. This system enables direct visualization of the dynamic processes of human tumor formation, angiogenesis, and cell invasion in living organisms at high resolution and in real-time.
Optical sectioning and 3D rendering in the present study reveals that GFP-labeled human tumor cells (MDA-453) form microtumors in the body of the zebrafish. They display various levels of cell invasion, angiogenesis and interact with newly formed vessels. The human metastasicgene RhoCis needed for the dynamic regulation of the actin/myosin cytoskeleton within the tumor cell to form protrusive structures - but exclusively at sites of vascular remodeling. For the ability to penetrate existing intact blood vessels, tumor cells need to secret an angiogenic factor (in this study VEGF), which induces vascular openings.
Tumors secreting VEGF displayed robust vessel sprouting and remodeling (figure 1). Images were 3D rendered and analyzed by using Imaris software. Quantitative 3-D computer analysis revealed a dramatic increase in the number of branch points, total vessel length, and vessel diameter of VEGF secreting MDA-435 tumors compared with MDA-435 control tumors developing in the absence of VEGF. Imaris FilamentTracer function was used for 3D measurements of vessel length, diameter, and determining the number of branch points contained within the 3D area imaged. Tumor cell size (diameter) was measured for the cells present in the z-stacks by using Imaris Spot function.
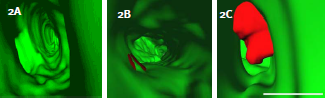
Figure 2:RhoC cooperates with VEGF to enhance cell Intravasation. A)3D reconstructions of tumor cells expressing RhoC, B)VEGF, or C)RhoC and VEGF. Fish vasculature=green, human tumor cells=red; scale bar=10μm.
Additionally when MDA-435 cells express VEGF and RhoC together they were able to protrude large membrane extensions (5μm or greater) into the vessel lumen (figure 2). The number of cells that displayed this phenotype was significantly higher compared with cells expressing RhoC or VEGF
This zebra fish model together with Image Analysis provides an efficient in vivo system for identifying mechanisms of cancer progression with the goal to unravel the mechanisms of human cancer and to develop cancer therapeutics.
Author: Prof. R. Klemke and colleagues, University of California at San Diego
Category: Andor Academy
