Hardware Solutions
Applications
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Immunology
Researchers from the Institute of Virology and Immunoprophylaxis and the University of Zürich, both in Switzerland, are using Imaris software to better understand interactions between dendritic cells and nanoparticulate vaccines. The nanoparticulate vaccines under study are based on synthetic virus-like particles and can efficiently induce adaptive immunity without the adjuvant usually required to enhance the recipient’s immune response. Dendritic cells are crucial for initiating and directing immune responses; understanding how synthetic virus-like particles interact with dendritic cells in vitro would provide important information that could be used to develop an efficacious vaccine in vivo.
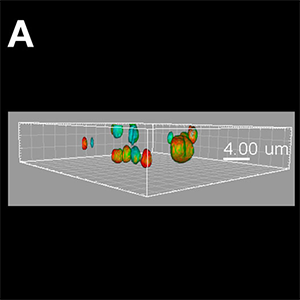
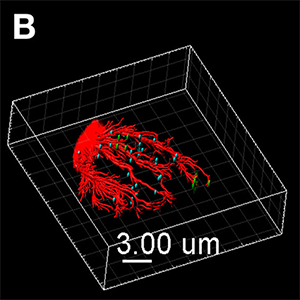
Figure (A) Synthetic virus-like particles (SVLP, green) co-localised with cholera toxin B (CTB, red) in lipid rafts, as well as association with CD9 (blue) containing rafts. The image shown is a Blend format. Figure (B) Once internalised, the SVLPs (green) remain peripheral, but associated with microtubules (red) extended into the region of the lamellipodium. These SVLP-containing vesicles remain distinct from the Major Histocompatibility Complex (MHC) Class II+ structures (blue). The image shown is a MIP (max) format, with the microtubules enhanced using the Filament Tracer module, and the SVLP-vesicles and MHC Class II-vesicles enhanced using the Spots module. Images courtesy of Kenneth C. McCullough.
The researchers are investigating how dendritic cells detect nanoparticulate vaccines, together with the roles played by particular cell receptors and the variety of endocytic processes associated with dendritic cells. In this study, they examined a nanoparticulate vaccine derived from synthetic lipopeptides that self-assemble into synthetic virus-like particles. The lipopeptides carry antigenic determinants that are reactive with B- and T- lymphocytes, and the vaccine can also be constructed to carry pathogen-associated molecular patterns that activate the dendritic cells.
The researchers imaged the interaction of dendritic cells with synthetic virus-like particles using a confocal scanning microscope and then analysed these images using Imaris. The Imaris software permitted a defined analysis and visual description of how dendritic cells endocytosed synthetic virus-like particles. “The Imaris software allowed accuracy of co-localisation through application of the co-localisation module, which gave both mathematical readouts and visual identification of co-localised voxels within the cellular structures,” Dr. McCullough says. “High definition of the visual description obtained for dendritic cell endocytosis of synthetic virus-like particles, and the associated cellular structures, was made possible through the powerful Filament Tracer module and by 3D visualization using Surface and Spots rendering
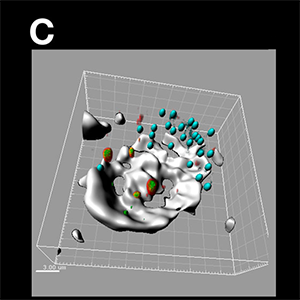
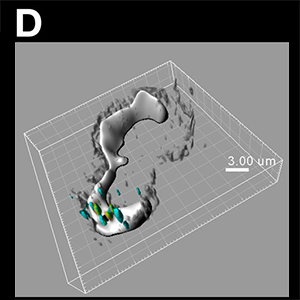
Figure (C) The peripheral SVLP-vesicles (green) co-localised with high molecular weight dextran-containing structures (red), demonstrating an association with macropinosomes (high molecular weight dextran accumulates in macropinosomes). They remained relatively peripheral behind the lamellipodium (depicted by the more intense and solid white/grey stain obtained using acquisition with Trans and image analysis with the Surface module), and distinct from the MHC Class II+ vesicles. The SVLP/dextran-vesicles (green/red/yellow) and MHC Class II-vesicles (blue) were enhanced using the Spots module, with the dextran Spots partially opaque to enhance visualisation of the green SVLPs with the red dextran; the whole image shown is a Normal Shading format. Figure (D) Peripheral SVLP-vesicles (green) observed at an earlier stage than in (C), co-localised with CTB (red) to show the GM-1 ganglioside lipid raft origin of the endocytic vesicles; at this earlier stage, the SVLP-vesicles have not yet co-localised with the macropinosomes in which high molecular weight dextran (blue) had accumulated. The application of the Surface module was as in (C), and the Spots module was applied to the SVLP/CTB-vesicles and dextran+ macropinosomes; the whole image shown is a Normal Shading format. Images courtesy of Kenneth C. McCullough.
The image analysis revealed details about the endocytic processes. They observed rapid interaction of the synthetic virus-like particles with the cell surface and slow internalisation involving GM-1 ganglioside-containing lipid rafts, polarizing to the leading edge of the cells. Structures containing the synthetic virus-like particles remained in peripheral intracellular locations for long periods, while slowing interacting with early endosomes and then macropinosomes. “Such characteristics of slow processing and retention in macropinosomes are important for the efficient processing of antigen for presentation to the adaptive immune system and stimulation of efficacious adaptive immune responses, relating to our current knowledge on how dendritic cells operate,” Dr. McCullough says. “This was reflected in the capacity of the synthetic virus-like particles to induce both humoral and cell-mediated immune responses in vivo, in murine and porcine models.”
Research Paper: Sharma R, Ghasparian A, Robinson JA, McCullough KC (2012) Synthetic Virus-Like Particles Target Dendritic Cell Lipid Rafts for Rapid Endocytosis Primarily but Not Exclusively by Macropinocytosis. PLoS ONE 7(8): e43248. doi:10.1371/journal.pone.004324.
Author: Drs. Kenneth C. McCullough and Rajni Sharma, Institute of Virology and Immunoprophylaxis; Dr. Arin G
Category: Case Study
