Hardware Solutions
Applications
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Neurobiology
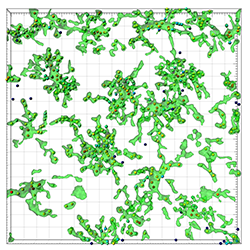
The figure shows a representative image of microglia in a LPS-injected mouse. The colour of the created Spots objects are is related to the distance of the Surfaces object.
Researchers led by Dr. Stephen F. Traynelis from Emory University School of Medicine, in collaboration with a team led by Katerina Akassoglou at the Gladstone Institute, recently used Imaris to track and image microglial responses to neuroinflammation in three dimensions. Their findings may help us to better understand the role of inflammation in neurodegenerative disease such as Alzheimer’s and Parkinson’s disease.
In a healthy body, microglia regularly scavenge the central nervous system looking for plaques, damaged neurons, or infectious agents that need to be cleared. However, when microglia are activated by neuroinflammation they change structure and function. These activated cells have been observed in much higher levels at sites of neurodegeneration in patients with neurodegenerative diseases, compared to healthy people.
To learn more about how microglia respond to tissue damage and cell death, the researchers acquired in vivo two-photon and confocal images of microglia in lipopolysaccharide (LPS)-treated mice, a model of peripherally-induced neuroinflammation. They turned to Imaris to create 3D reconstructions that let them examine the complex morphology of microglia and also to track the microglia, which are highly motile cells, over time.
Quantifying motility
To quantify the process motility patterns of microglia in the absence of tissue damage, the researchers created three-dimensional reconstructions of volumes imaged with two-photon microscopy and used Imaris to detect objects with a diameter larger than 2 microns. Then using Imaris Track Autoregressive Motion GapClose algorithm, these objects were tracked over time to determine the average length and speed of movement during the entire recording.
They also studied the dynamics of microglial processes that were activated by LPS. For this, they acquired confocal Z-plane stacks of primary microglial cells every 30 s and then analyzed these with Imaris. After subtracting the background, they generated Imaris Surface objects, three-dimensional representations of the cells from the z-stacks. To measure changes in cell ramification, the objects surface area-to-volume ratio was calculated at each time point.
The team then extended their findings to microglial response to tissue damage in acute brain slices. “Confocal imaging and reconstruction of the slices with Imaris provided excellent spatial and temporal resolution and tracking capabilities to study cell motion,” said Dr. Stefka Gyoneva, a member of the research team. “In addition, our analysis of microglial process movement in slices shows that brain slices provide a useful system for analysis of microglial function.”
Inflammation-induced changes
Tracking the movement of microglial processes over time showed that microglia in LPS-injected mice have hypermotile processes in the absence of tissue damage, but display a slower response to the damage after it is experimentally induced. “Our findings show that inflammation changes the morphology and motility of microglia,” said Dr. Gyoneva. “Since damage in the brain through the natural death of neurons occurs on a daily basis, microglia that are activated by neuroinflammation may be inefficient in responding to and clearing the damage, which could ultimately impair the function of surrounding neurons.”
Author: Dr. Stephen F. Traynelis, Dr. Stefka Gyoneva, and colleagues, Emory University School of Medicine
Category: Case Study
