Hardware Solutions
Applications
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Development
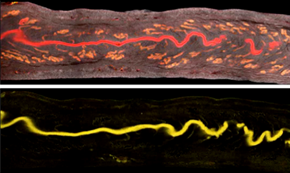
The researchers used the Imaris Channel Arithmetic Matlab function to subtract the glandular signal (bottom) from the total epithelial signal (top). Reprinted from Arora et al., Development 143(23): 4749-4754.
Researchers led by Dr. Ripla Arora of the University of California in San Francisco recently developed a method that uses Imaris to visualize the lining of the mouse uterus. Their new method could help scientists better understand the 3D architecture of the endometrium in normal and abnormal conditions, which might one day help improve artificial reproductive technologies and treatment of infertility.
Although it has been long known that the uterine glands are crucial for establishing a pregnancy, how these glandular secretions reach the embryo is not well understood. Using the new imaging method, the researchers document, for the first time, the organization of the mouse uterine luminal and glandular structure in three dimensions. They show that the uterine lumen undergoes organized folding and that the uterine glands reorganize in preparation for attachment of the incoming embryo.
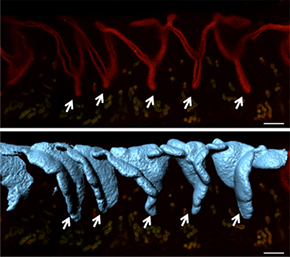
With Imaris Surface, the researchers could see that the 2D crypts in Z slices (top) are actually 3D folds (bottom). Reprinted from Arora et al., Development 143(23): 4749-4754.
“We initially tried Imaris because of its ability to handle large file sizes, but once we started using the software we realized its great potential in helping us analyze complex data in 3D,” said Dr. Arora, a postdoctoral fellow in Dr. Diana Laird’s laboratory. “Another major advantage of Imaris is the ability to deconstruct the data using scripts written in Matlab. We used pre-integrated Matlab scripts as well as a custom script for our data analysis.”
Analyzing 3D Surface Curvature
For the study, the researchers used confocal microscopy to image mouse uteri by acquiring Z-stacks that were 7 microns apart. In the absence of a marker to label the luminal epithelia, they used the Imaris Channel Arithmetic function (XTension) to subtract the glandular signal from the total epithelial signal. This produced two channels, each with either the luminal or glandular signal. The researchers then used the Imaris Surface model to create surfaces for the lumen and the glands.
After observing that the uterine lumen undergoes dynamic folding as it prepares for implantation, the researchers worked with the Bitplane Customer Support Team to write a custom XT script to measure the curvature of a 3D surface to measure the folding. (Script available at: http://open.bitplane.com/tabid/235/Default.aspx?id=130).
Preparing for the Embryo
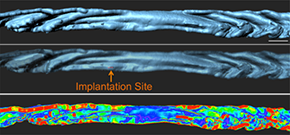
A custom script allowed measurement of the curvature for a 3D surface and thus measurements of the folds. Reprinted from Arora et al., Development 143(23): 4749-4754.
From the image analysis, the researchers inferred that structures known to hold the incoming embryo, which are referred to as embryonic crypts in 2D histological sections, coincide with folds in the 3D surface reconstructions. This suggests that the uterine lumen folds to create a holding place for the embryo before the embryo attaches to the luminal epithelium.
The researchers also saw that the glandular ducts elongate and bend towards the site of implantation. Utilizing Imaris Measurements and Orthoslicer in different axes, they measured the angles that the glandular ducts make with the uterine lumen, thus confirming the bending of the ducts. Finally, they used ImarisVantage to create a montage of the individual glands.
“The angle measurements and montage showed that in non-pregnant uteri, the glandular trees have short ducts and branched buds, whereas at the time of implantation some glandular trees have ducts that are elongated with seemingly fewer buds,” said Dr. Arora. “We hypothesize that the glandular reorganization towards the site of embryo attachment is instrumental in delivering glandular secretions to the embryo.”
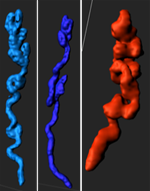
The researchers observed glandular branching, coiling, and duct elongation at the time of implantation. Reprinted from Arora et al., Development 143(23): 4749-4754.
Studying genetic mutants and human endometrium
The researchers are currently working to understand the mechanisms that lie upstream of the uterine luminal folding to form crypts that hold the embryo prior to attachment. They are also investigating 3D architecture for genetic mutants with known defects in embryo implantation and the functional consequences of glandular reorientation.
“We believe that 3D spatial localization of glandular secretions will help determine how the embryo accesses these secretions,” said Dr. Arora. “Applying this method on genetic mutants with known defects in uterine epithelial development and embryo implantation will help reveal the role of signaling in the establishment of the observed uterine topology and the significance of these 3D structure changes. We can also use this method to visualize epithelia in a biopsy specimen from human endometrium.”
Videos
Author: Dr. Ripla Arora, Dr. Diana Laird and colleagues, University of California, San Francisco
Category: Case Study
