Hardware Solutions
Applications
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Cell Biology
Studies have shown that people who consume excess dietary iron have an increased risk of colorectal cancer. To better understand how iron affects colorectal cancer cells and the development of cancer, researchers led by Dr. Owen J. Sansom from the Beatson Institute for Cancer Research in Glasgow, UK, used Imaris software for 3D imaging.
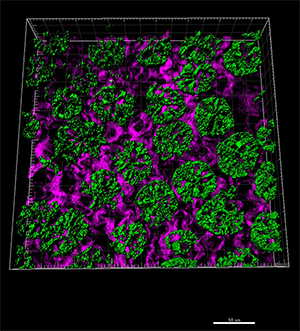
This section of intestine is from a mouse fed a high iron diet. Lgr5-positive stem cells labeled with GFP (green) were imaged with multiphoton microscopy. The purple background shows second harmonic generation signals from collagen. An associated movie shows the volumes for the GFP.
“Our work provides a novel link that colorectal cancer cells require iron from the very earliest stages of the disease. Conversely, increasing iron levels also promotes tumourigenesis,” Dr. Sansom said. “This is consistent with studies showing that diets high in red meat increase colorectal cancer risk.”
Colon tissue has compartments called crypts that contain stem cells. Normally, the stem cells produce new specialized cells, but if this process is interrupted the stem cells can produce enough malignant cells to cause a tumor. The researchers used mice with the adenomatous polyposis coli (Apc) gene deleted only in their stem cells. Mutations in the Apc tumor suppressor gene commonly lead to colorectal cancer. These mice, which rapidly develop intestinal tumors, received a control diet, a diet containing excess iron, or a diet deficient in iron.
The investigators then used multiphoton microscopy to image sections of intestinal tissue from these mice. With Imaris they observed and modeled intestinal crypt stem cells in three dimensions and quantified the physical effects of different diets on the stem cells. “Being able to accurately reconstruct the fluorescence data into real three dimensional objects with unique parameters allows us to ask a much wider range of questions and get meaningful results,” Dr. Sansom said.
Specifically, they used Imaris to measure the volume occupied by GFP expression that marked the stem cell specific marker gene Lgr5. This let them determine how the different diets effected intestinal crypts and tumor progression. Their analyses showed that depletion of iron in the colon lumen strongly suppressed development of intestinal tumors in the mice. However, lowering the overall levels of circulating iron did not have this effect. Higher levels of luminal iron strongly promoted tumor development.
The findings from this research could have important implications for the diets of patients who have a high risk of developing colorectal cancer. The researchers next aim to study how iron drives changes in colorectal cancer. They believe that iron acts in part through activation of NF-KB signaling and by increasing levels of reactive oxygen species. They also want to see if agents that collate iron (and hence lower levels) can also suppress tumor development, which might lead to a potential therapy. They plan to investiagte the impact of these collating agents on Lgr5 positive stem cells using Imaris.
Research Paper: Radulescu S, Brookes MJ, Salgueiro P, Ridgway RA, McGhee E, Anderson K, Ford SJ, Stones DH, Iqbal TH, Tselepis C, Sansom OJ. Luminal iron levels govern intestinal tumorigenesis after Apc loss in vivo. Cell Rep. 2012 Aug 30;2(2):270-82. doi: 10.1016/j.celrep.2012.07.003.
Author: Dr. Owen J. Sansom, Beatson Institute of Cancer Research, and colleagues.
Category: Case Study
